PSMA as a relevant diagnostic and therapeutic target for PSMA prostate cancer
PSMA (prostate-specific membrane antigen) is a protein abundantly expressed on the surface of prostate cancer cells, making it an ideal target for diagnostic imaging and therapeutics4. PSMA-PET scans use radiolabeled ligands that bind to PSMA, allowing for the detailed visualization of distant lesions from prostate cancer throughout the body, making it a valuable tool for staging PSMA prostate cancer at diagnosis with precision, discovering difficult-to-detect micro-metastatic disease, identifying tumor locations responsible for biochemical recurrence, and assessing response to PSMA PET treatment.
PSMA-PET in clinical guidelines
Guidelines from leading organizations, such as the American Urological Association (AUA), European Association of Nuclear Medicine (EANM), and the National Comprehensive Cancer Network (NCCN), have recognized the importance of PSMA-PET in the management of PSMA prostate cancer1,3. These guidelines highlight the role of PSMA-PET in improving diagnostic accuracy and guiding therapeutic decisions in PSMA clinical trials, highlighting its significance in current oncology practice.
From PSMA-PET imaging to actionable predictions in PSMA prostate cancer
Quibim has developed robust capabilities in its PSMA prostate cancer pipeline to extract imaging biomarkers and radiomics features from PSMA-PET images and transform them into actionable predictions.
After rigorous standardized imaging quality control procedures, we meticulously perform a comprehensive segmentation of the total tumor burden in patients with PSMA prostate cancer. We extract imaging biomarkers and radiomics features from these segmented lesions, including shape characteristics, first, second, and higher-order features, PSMA total lesion (TL) count, PSMA total volume (TV), and lesion uptake. Additionally, we calculate parameters associated with the normalized standardized uptake value (SUV), such as the SUVmax, SUVratio, SUVmean, SUVpeak and its percentiles. We use this quantitative data, along with additional clinical and molecular data (if available, and if needed), to develop AI-driven solutions capable of quantitatively assessing the efficacy and specificity of radiolabeled ligands in targeting PSMA within the tumor microenvironment. These solutions aim to predict important patient outcomes, including risk of metastasis, response to PSMA PET therapy , and overall survival (OS), among others.
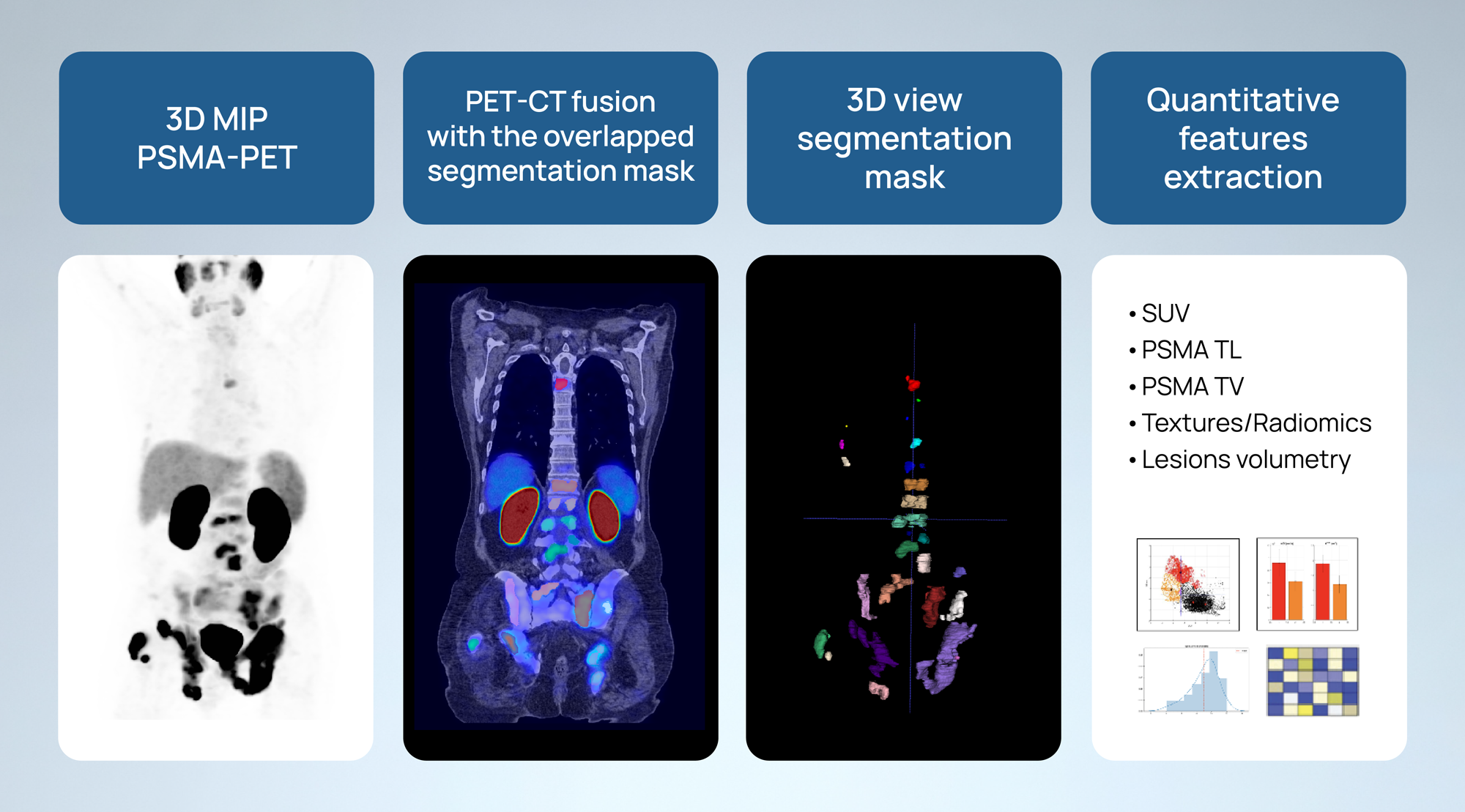
This innovative approach has not only enhanced our predictive capabilities but also aligns with and contributes to the growing PSMA PET imaging market. Numerous studies have demonstrated the significant prognostic and diagnostic value of quantitative PSMA-PET, highlighting the transformative potential of our methodology:
- A higher SUVmean value typically indicates higher density of the PET imaging target, which can be associated with malignant processes. SUVmean is widely used for characterizing tumors, assessing response to therapy, and monitoring disease progression. In several studies, baseline SUVmean obtained from PSMA-PET scans emerged as an effective predictor of treatment response to radioligand therapies targeting PSMA-positive tumors. Typically, higher SUVmean levels at baseline correlate with better treatment response and longer progression-free survival (PFS) and overall survival (OS)5.
- In a recent PSMA clinical trial of patients with metastatic castration-resistant prostate cancer (mCRPC), PSMA TL was found to be a prognostic biomarker, “significantly differentiating between [partial response] PR, [stable disease] SD, and [disease progression] PD” for patients treated with [177Lu]Lu-PSMA-617 radioligand therapy (RLT)2.
Using quantitative PSMA-PET in clinical studies
When collaborating with pharmaceutical partners, we explore the quantitative and radiomics features extracted from patient images from clinical trials to perform statistical analyses correlating those features with clinical data and outcomes, such as PSA50, duration on treatment, PFS, OS, among others, based on the primary and secondary objectives of the trial. Since its inception, Quibim has invested expertise and care into solving the complex challenges associated with non invasively assessing PSMA prostate cancer for point-in-time assessments, longitudinal monitoring in research and PSMA clinical trials, as well as developing AI-based predictive and prognostic models.
Our approach enables biopharma companies to support breakthrough therapy designation, significantly improve patient stratification for screening, increase the likelihood of securing funding for future PSMA clinical trial phases, expedite regulatory clearances, and facilitate the development of imaging-based companion diagnostics. Furthermore, by optimizing the use of PSMA-PET imaging data, we help enhance the precision of clinical trial outcomes, ultimately leading to more personalized and effective treatment for patients. This streamlined process not only accelerates drug development but also reduces costs and time-to-market, benefiting both the industry and patients alike.
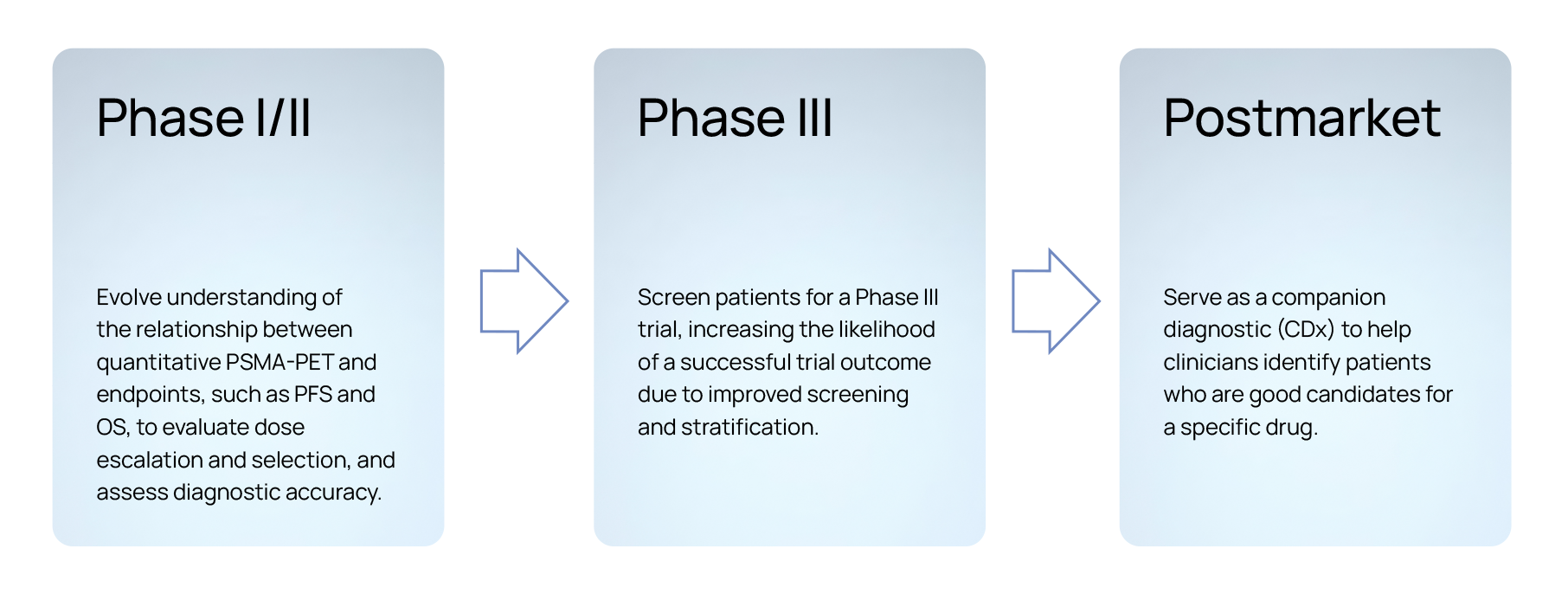
Setting a new benchmark for PSMA-PET
Quibim is proud to be furthering research in this field, applying our skills developed with other types of precision imaging, such as FDG-PET, and translating them to PSMA-PET. In addition to expertise in combining quantitative imaging with AI to improve diagnosis and develop predictive models for PSMA prostate cancer, our team brings significant experience through our flagship certified QP-Prostate® solution, having participated in dozens of grant-based research projects and has partnered with several of the top-20 biopharma companies.
Through the meticulous analysis and interpretation of imaging biomarkers and radiomics features, Quibim aims to set a new standard in the utilization of PSMA-PET in clinical trials, contributing to the PSMA PET imaging market and bringing effective and safe new PSMA PET therapies to the patients who need them.
References
- “Advanced Prostate Cancer,” American Urological Association. [Online]. Available: https://www.auanet.org/guidelines-and-quality/guidelines/advanced-prostate-cancer. [Accessed: 17, May, 2024].
- C. Burgard, C. Hein, A. Blickle et al., “Change in total lesion PSMA (TLP) during [177Lu]Lu-PSMA-617 radioligand therapy predicts overall survival in patients with mCRPC: monocentric evaluation of a prospective registry,” Eur. J. Nucl. Med. Mol. Imaging, vol. 51, pp. 885–895, 2024. [Online]. Available: https://doi.org/10.1007/s00259-023-06476-x.
- W. P. Fendler, M. Eiber, M. Beheshti et al., “PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0,” Eur. J. Nucl. Med. Mol. Imaging, vol. 50, pp. 1466–1486, 2023. [Online]. Available: https://doi.org/10.1007/s00259-022-06089-w.
- W. I. Luining, M. C. F. Cysouw, D. Meijer, N. H. Hendrikse, R. Boellaard, A. N. Vis, and D. E. Oprea-Lager, “Targeting PSMA Revolutionizes the Role of Nuclear Medicine in Diagnosis and Treatment of Prostate Cancer,” Cancers (Basel), vol. 14, no. 5, art. no. 1169, Feb. 2022. [Online]. Available: https://doi.org/10.3390/cancers14051169.
- R. Eisazadeh, S. A. Mirshahvalad, G. Schwieghofer-Zwink et al., “Pre-treatment 68 Ga-PSMA-11 PET/CT Prognostic Value in Predicting Response to 177Lu-PSMA-I&T Therapy and Patient Survival,” Mol. Imaging Biol., vol. 26, pp. 360–369, 2024. [Online]. Available: https://doi.org/10.1007/s11307-024-01900-6.
Author: Sarah Vizel, Project Manager at Quibim.
