The challenge
Axial spondyloarthritis (axSpA) is an immune-mediated inflammatory disease that primarily affects the axial skeleton, causing significant pain and disability. With an estimated prevalence ranging from 0.3% to 1.4% worldwide, axSpA can be classified into radiographic and non-radiographic axSpA based on whether the condition can be detected with ionizing radiation. Magnetic resonance imaging (MRI) has revolutionized axSpA diagnosis, improving the likelihood of identifying non-radiographic disease; this allows patients to receive optimal targeted treatments which improves long term outcomes. However, the definition of a “positive MRI” remains contested, with notable inter- and intraobserver variability depending on radiologist’ expertise, which causes diagnostic delays (average of 6.7 years from the presence of the first symptoms) and underdiagnoses. As a result, 16.4% of patients with chronic lower back pain (CLBP) are misdiagnosed, despite actually having axSpA1, and, on average, axSpA diagnoses are delayed by 6.7 years2.
This situation creates challenges in ensuring the accessibility and effectiveness of monoclonal antibodies for treating axSpA, creating uncertainty that can indirectly impact the ability to optimize patient outcomes and sustain long-term advancements in care. Therefore, new tools for homogenizing and automating the axSpA diagnostic procedure are urgently needed.
The solution
Coupling AI to MRI holds the potential to develop a non-invasive tool that automatically detects axSpA through the extraction of patterns that cannot be perceived by the human eye. Quibim’s solution will offer a systematic method for interpretation of imaging data through QP-Insights® platform that radiologists and rheumatologists can use for axSpA diagnosis.
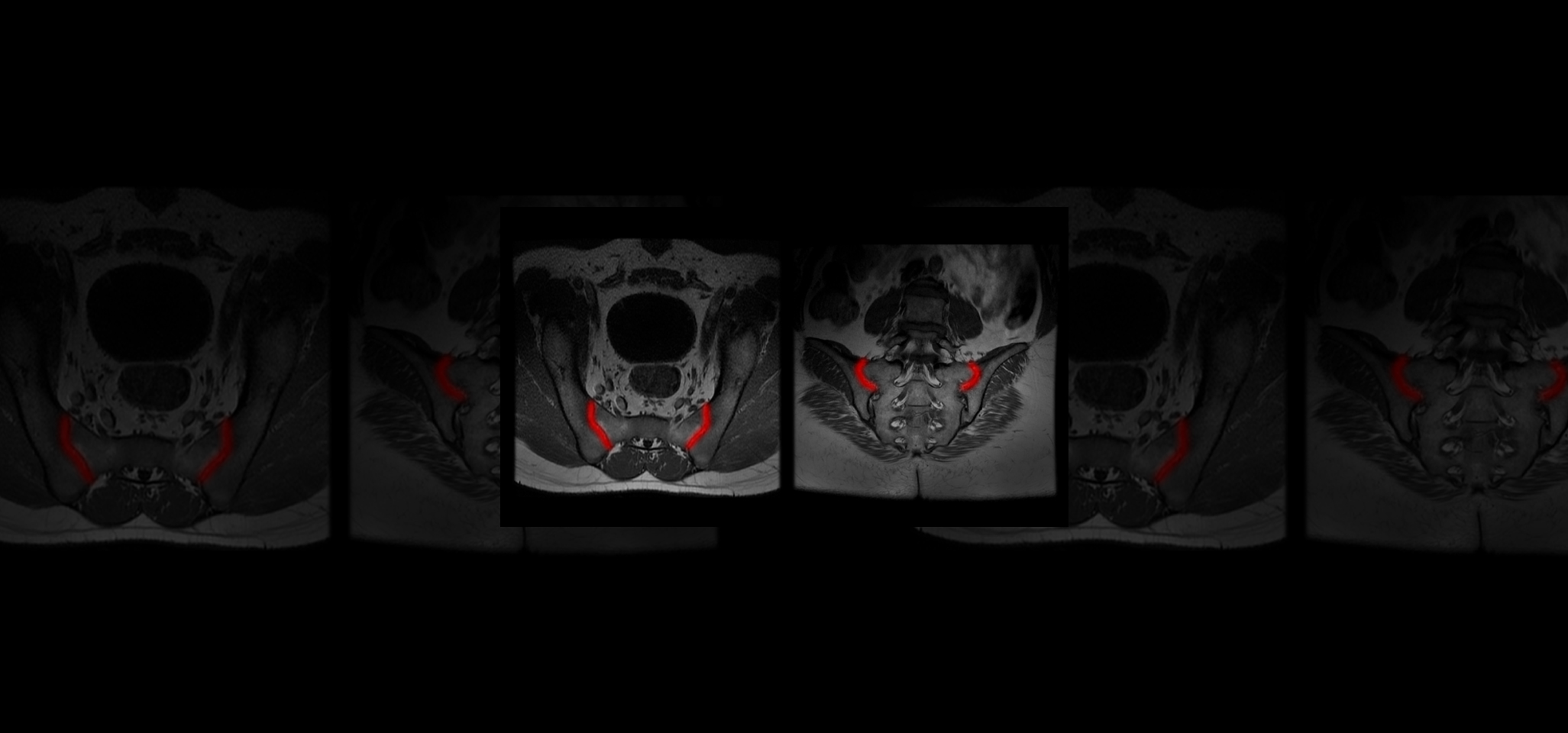
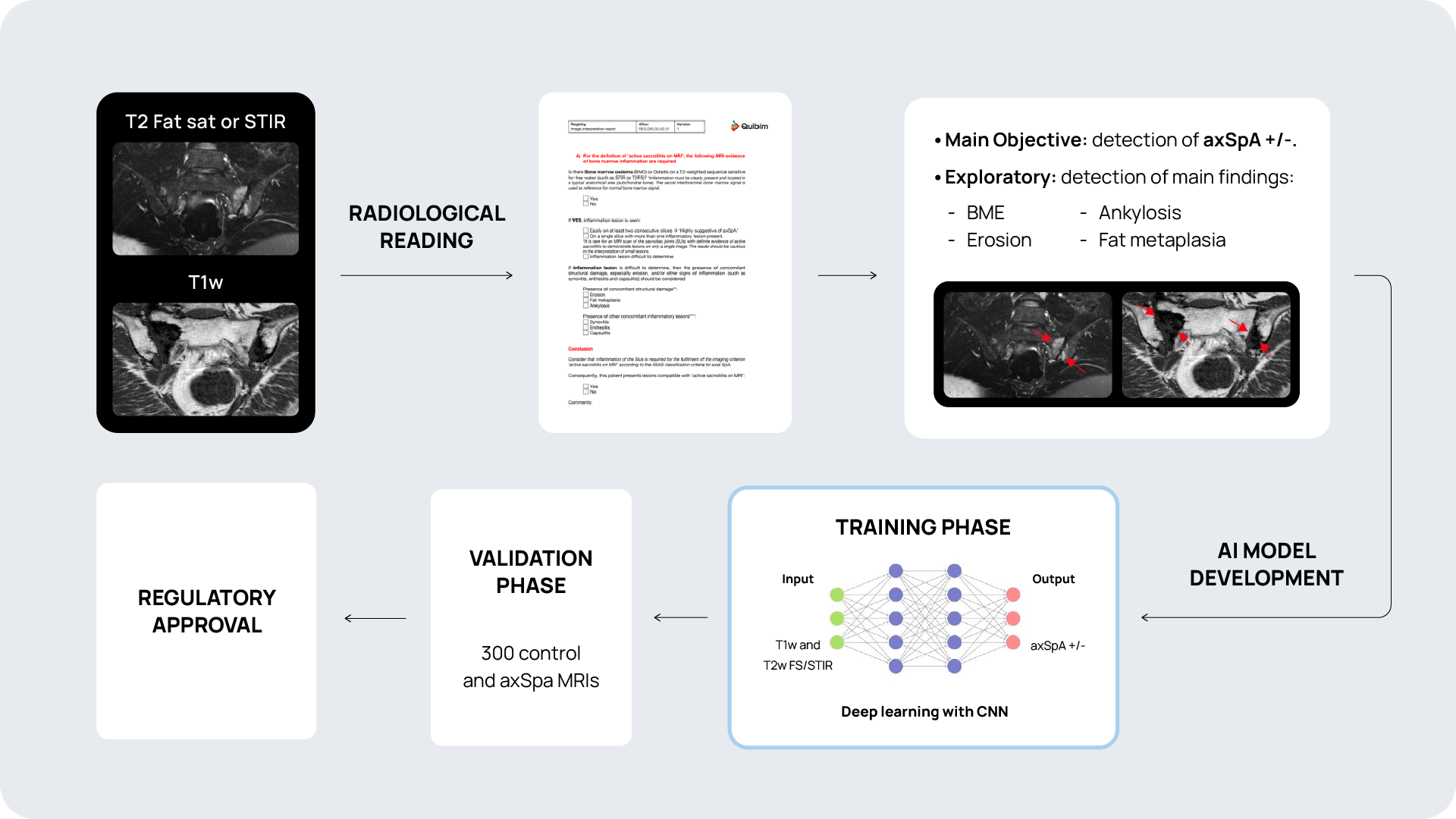
Quibim, in collaboration with a top-tier biopharmaceutical company, is conducting a retrospective observational real-word study with data from six sites world-wide (NCT06591481). The training dataset consists of 600 MRIs of the sacroiliac joint (SIJ) including a T1-weighted and STIR and/or T2 fat saturated sequence in coronal-oblique planes acquired at diagnosis and follow-up. For the validation process, an external sample of 200-300 MRIs with and without axSpA are being collected. QP-Insights® platform is being used for the image collection and processing. A double reading according to the Assessment of Spondylarthritis international Society (ASAS) criteria is being performed by radiologists with 12+ years of experience and a third reader for adjudication. The reading process consists of the classification into axSpA +/- and identification of the main findings: bone marrow edema (BME), ankylosis, erosion and fat metaplasia. An artificial intelligence (AI) based classification model will be developed to categorize patients as axSpA-positive (axSpA+) or axSpA-negative (axSpA-). Additionally, as an exploratory objective, the model will further stratify patients based on the main findings identified.
This tool represents a step forward in enhancing patient care by equipping clinical specialists with both advanced treatment options and an AI-powered tool to support accurate and timely disease diagnosis, addressing gaps in accessibility and precision.
The outcome
Quibim is designing and creating a new tool with an already-existing product-market fit and with a unique value proposition.
This tool enables the biopharmaceutical company to reduce variability in revenue across hospitals, which is primarily tied to the radiologist’s expertise in identifying axSpA, while providing a standardized approach to MRI interpretation. It also represents a digital transformation in the management of monoclonal antibody therapies, offering rheumatologists both the drug and an AI-powered diagnostic tool to identify axSpA more effectively. Ultimately, this innovation has the potential to significantly enhance patient outcomes and quality of life.
References
-
Van Hoeven et al. Arthritis Research & Therapy (2017) 19:143
-
Zhao SS et al. Rheumatology (Oxford)(2021) 60:1620–1628
Related case studies
-
Empowering neuroblastoma risk stratification through AI algorithms
Read more
-
Prognostic value of genetic alterations and 18F-FDG PET/CT imaging features in diffuse large B cell lymphoma
Read more
-
CT-based clinical-radiomics model to predict progression and drive clinical applicability in locally advanced head and neck cancer
Read more
