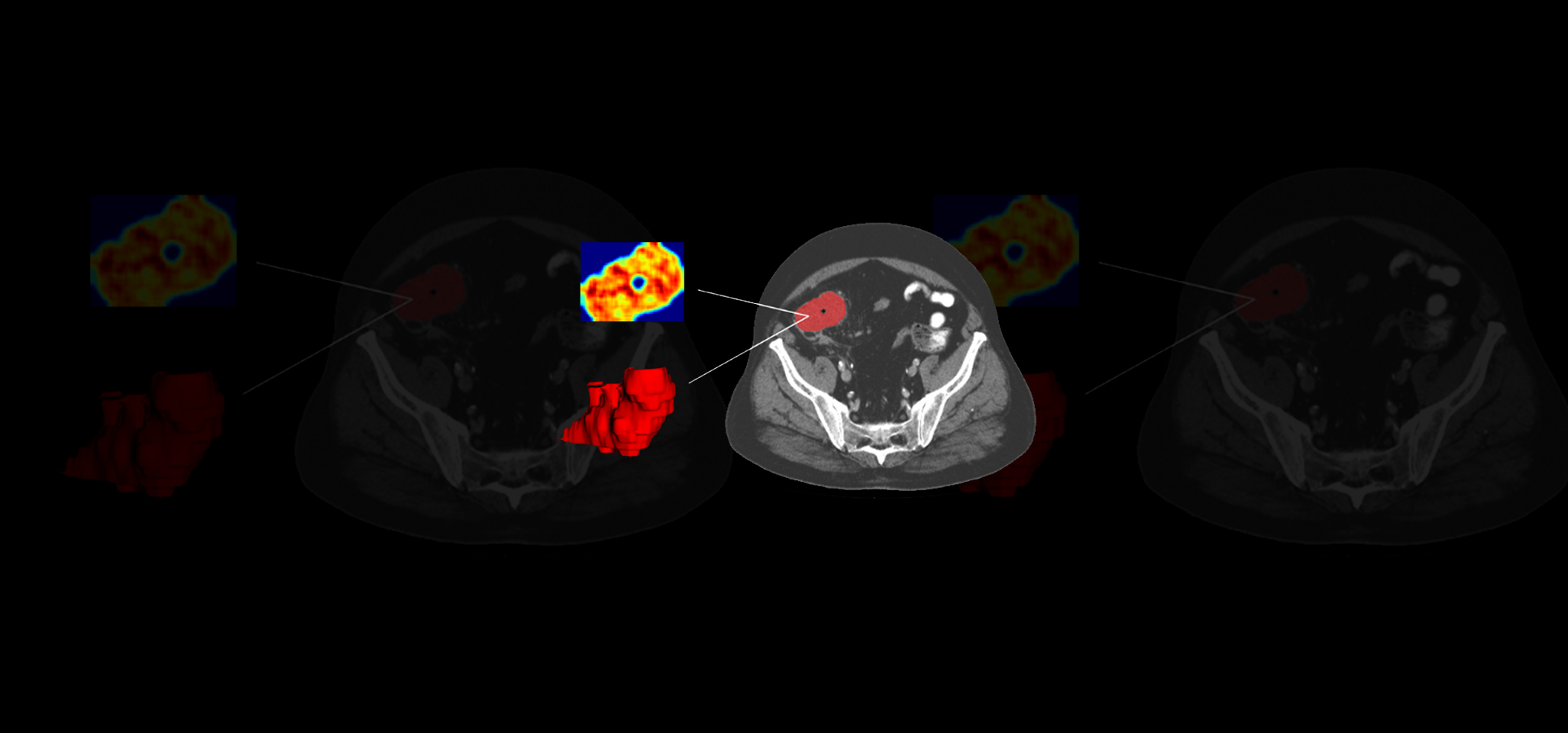
The challenge
A prestigious Health Research Institute that pioneered liquid biopsy analysis in colon cancer patients was interested in creating predictive and prognostic biomarkers to identify patients at high risk of relapse and predict the effect of adjuvant therapy.
Traditional methods of measuring and staging minimal residual disease (MRD) are invasive procedures. New prognostic biomarkers, such as liquid biopsy tests, even though they may improve MRD estimation, still provide limited sensitivity to identify patients at high risk of relapse.
However, the seamless integration of liquid biopsy with radiomics and deep features extracted from the CT scans (standard-of-care [SoC] data) could offer a new multi-omics tool to monitor and diagnose tumors at an early stage, providing relevant insights in the prognostic of relapse and patient outcomes.
The solution
Following a collaborative design, we collected retrospective multi-center real-world data (RWD) from a large sample of patients with diagnoses confirmed by traditional methods. The RWD included clinical data, liquid biopsy, and SoC CT scans. A 3D segmentation of the tumoral tissue was performed, and textures, deep features, and fractal dimensions parameters were extracted. Once all of these quantitative imaging biomarkers were obtained, several AI-based classification models were trained combining the imaging features with liquid biopsy and clinical data to predict early relapse in patients and classify patients with low and high risk. The analysis pipeline generated was integrated into QP-Discovery® platform, creating an end-to-end AI tool.
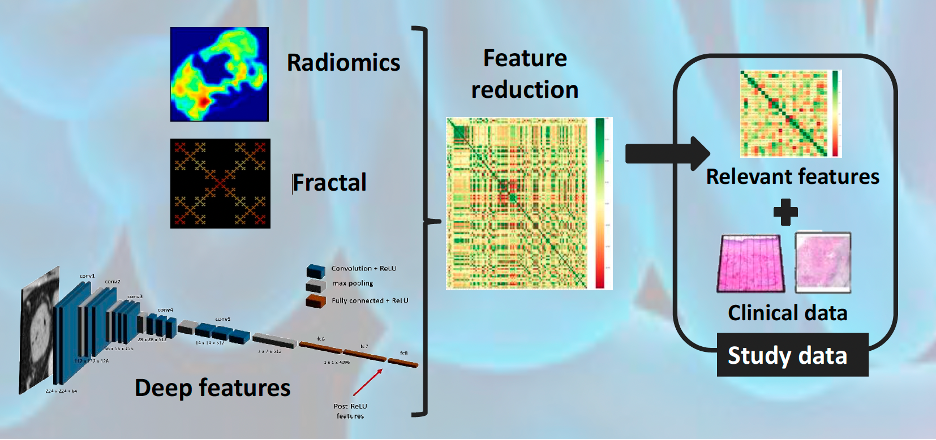
The outcome
The innovative potential of this new approach conducted to three primary outcomes:
- The identification of the imaging signature for the early detection of relapse in localized colon cancer,
- The development of a stratification tool to differentiate between low and high-risk colon cancer patients,
- The creation of predictive models to measure the effect of adjuvant therapy that tests the antitumor efficacy of different agents, which will result in new exploitable therapeutic targets.
By merging clinical data with radiomics, fractal, and deep features, the AI model provided a high and increased accuracy of 87.9% (CI 95%: 67.7–100) when compared to clinical variables alone (60%, CI 95%: 30–90) in the prediction of relapse. The most accurate AI-based model is expected to be obtained by combining radiomic features with RWD to predict potential patients to be relapsed, leading to the selection of personalized treatments that improve response over current standard therapies.
Reference:
America Bueno Gomez et al. A clinico-imaging predictive artificial intelligence model of relapse in colon cancer using baseline CT scans. Oral comunnication accepted at ESMO 2023. 20-23 October. Madrid, Spain.
Related case studies
-
Drug revival: Prediction of treatment response to immunotherapy in NSCLC
Read more
-
Post-approval: Identifying patients suffering from axial spondyloarthritis (axSpA) at an early stage, candidates for an approved drug
Read more
-
CT-based clinical-radiomics model to predict progression and drive clinical applicability in locally advanced head and neck cancer
Read more
